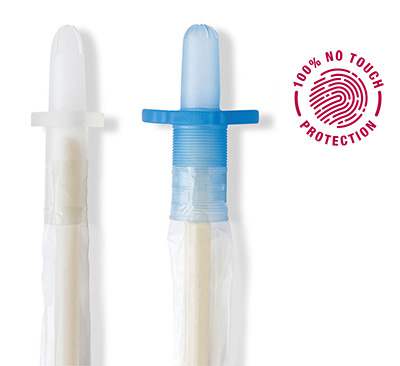
VaPro catheters are designed to help protect patients from germs throughout the entire catheterization process
Watch the videos below to learn how the VaPro catheter’s protective tip and sleeve protect patients from pathogens and hear what VaPro catheter users have to say about it.
The combination of protective tip and sleeve are why all VaPro™ catheters provide 100% No Touch Protection.
The protective tip
- Helps shield the sterile catheter during insertion from bacteria located within the first 15mm of the distal urethra
- Helps reduce the risk of carrying bacteria into the urinary tract
The protective sleeve
- Allows for catheter to be gripped anywhere
- Provides a barrier that helps keep germs away from the catheter

Experience the ease of use and comfort of our HydraBalance™ Lubricating Technology
Made with naturally sourced ingredients, its unique combination of hydrophilic coating and hydration fluid creates a smoothly gliding cushion that not only helps protect the urethra, but also provides smooth and effortless insertion and withdrawal for a more confident and natural catheter experience.
VaPro™ No Touch Intermittent Catheters: A Superior Choice for Complete Bladder Drainage
Sediment and mucus left in the bladder can be problematic. Watch and learn how VaPro™ Intermittent Catheters, with two optimally designed round eyelets, are designed for complete bladder drainage with 100% No Touch Protection.


VaPro™ catheters are easy to use
Ready to use right out of the package with no extra steps required

Designed with smooth rounded eyelets to enhance user comfort during insertion and withdrawal
Packaging designed with finger holes to be easy to open

Pocket-sized packaging for easy transportation and discreet out of home usage
A broad portfolio
No matter where you are—at home or on the go—VaPro™ catheters offer you many options to fit your lifestyle and meet your needs.
Standard system
- VaPro™—Catheter with protective tip and sleeve
- VaPro Pocket™—Discreet pocket-sized packaging
The voice of VaPro™ users
The latest user satisfaction study explored real-world preferences associated with using VaPro™ catheters. 25 participants from 5 countries (UK, Germany, France, the Netherlands, and the US) were interviewed to assess the perceived advantages of VaPro™ catheters compared to other intermittent catheters. All interviewees had experiences with competitive intermittent catheters and changed to VaPro™ catheters.

- Participants reported a high level of satisfaction with VaPro™ catheters enabling them to overcome issues they had encountered with other intermittent catheters.

- Some participants reported that VaPro™ catheters had a positive effect on their quality of life.

- Participants find VaPro™ catheters to be hygienic and easy to use

- 50% of participants reported less frequent UTIs after changing to VaPro™ catheters
Protective tip — proven pathogen protection
The protective tip works to reduce the risk of bacteria being carried into the urinary tract during the insertion process. This illustration demonstrates its effectiveness.

The catheter is advanced into the protective tip, and then the protective tip is inserted into the urethra.
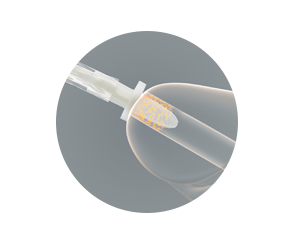
The protective tip helps prevent the catheter from coming into contact with the bacteria that are predominantly located in the first 15 mm of the distal urethra.
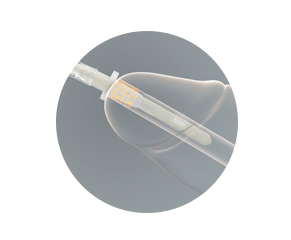
Read the summary of clinical and laboratory evidence demonstrating that the protective tip has been shown to reduce the introduction of bacteria to the urinary tract. Reducing the introduction of bacteria may help reduce the risk of catheter associated UTIs.
Protective sleeve — proven pathogen protection
The VaPro™ catheter sleeve material passes the ASTM F1671 test, providing assurance that it protects against pathogens that may cause UTIs.

Viruses
(smaller in size)
Bacteria
Fungi
Parasites
(greater in size)
The American Society for Testing and Materials (ASTM) is an international standards organization that develops and publishes voluntary consensus technical standards tor a wide range of materials. ASTM has also conducted and published approximately 6000 test methods to date.
ASTM F1671 is a pass or fail test designed to determine if protective materials prevent the transmission of blood-borne pathogens.
VaPro™ catheter sleeve material was tested. In the test, one side of the sleeve material was exposed to a solution containing a type of virus called bacteriophage. Results showed that the viruses did not pass through the sleeve material.
Read the summary of an additional laboratory study which showed that intermittent catheters with the sleeve helped reduce the potential for external contamination during preparation and insertion of an intermittent catheter. This reduction may help reduce the risk of bacteria entering the bladder.
Our Products
Learn more about the wide variety of VaPro™ 100% No Touch Protection products.







