
CeraPlus™ Skin Barrier with Remois Technology* is Shown To Have a Positive Impact On Stoma-related Health Care Costs
The ADVOCATE study brings clinical evidence to help support decision making.
Fewer than 1 in 5 ostomy patients seek treatment for PSCs4
Peristomal skin complications (PSCs) lower health-related quality of life and raise cost of care.1-3 Yet, often they are considered just part of life with a stoma.

ADVOCATE Study design
- 153 participants
- Double-blinded study
- 25 sites in a multicenter, multinational trial
- Adaptive design planned for two interim analyses
Results
Lower cost of care for CeraPlus™ skin barrier users over a 12-week period (14% relative reduction)5

More CeraPlus™ skin barrier users resolved skin complications within a four-week period5

15% fewer PSCs in the CeraPlus™ skin barrier group5
More CeraPlus™ skin barrier users "very satisfied" with overall performance5
CeraPlus™ Skin Barrier
Helps maintain healthy skin from day one.
Ceramide-infused formulation helps protect against damage, dryness, and itching.
The CeraPlus™ skin barrier comes in a range of fit options including one-piece, two-piece, flat, firm convex, soft convex, tape border, and tapeless.
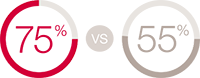
CeraPlus™ skin barrier is significantly less disruptive to the skin compared to SenSura® Mio6 **
Fit + Formulation is the inspiration behind CeraPlus™ skin barrier
![]()
*Remois is a technology of Alcare, Co., Ltd.
**SenSura Mio is a trademark of Coloplast A/S and its affiliates
40.5% had PSCs in treament group vs 55.4% in control, p=0.069. (Not statistically significant)
References:
- Persson E, Gustavsson B, Hellstro A, Lappas G, Hulten L. Ostomy patients' perceptions of quality of care. J Adv Nurs. 2005;49(1):51-58.
- Gray M, Colwell JC, Doughty D, et al. Peristomal moisture associated skin damage in adults with fecal ostomies: a comprehensive review and consensus. J Wound Ostomy Continence Nurs. 2013;40(4):389-399.
- Coons SJ, Chongpison Y, Wendel CS, Grant M, Krouse RS. Overall quality of life and difficulty paying for ostomy supplies in the Veterans Affairs ostomy-related quality of life study. Med Care. 2007;45(9):891-895.
- Nybaek H, Band KD, Norgaar LT, Karlsmark T, Jemec GB. Skin problems in ostomy patients: a case control study of risk factors. Acta Derm Venereol. 2009;89(1):64-67.
- Colwell J, Pittman J, Raizman R, Salvadalena G. A Randomized Controlled Trial Determining Variances in Ostomy Skin Conditions (ADVOCATE). J Wound Ostomy Continence Nurs. 2018;45(1):37-42.
- Grove G. Houser T, Sibbald G. Salvadalena G. Measuring epidermal effects of ostomy skin barriers. Skin Res Technol. 2019;25:179-186. https://doi.org/10.1111/srt.12630




